How to Test for Varroa Mites
Varroa mites (Varroa destructor) are an ectoparasite that feed on the fat body and hemolymph of honey bees and are vectors of diseases such as Deformed Wing Virus. If left unmanaged and untreated in a colony, a varroa mite infestation can result in the collapse of the whole colony and in some cases, the whole apiary. Regularly testing for varroa mites is an essential protocol throughout the year. In British Columbia, it is recommended to test your colonies for mites every 4-6 weeks from the beginning of the beekeeping season (spring) to the end (fall). Outlined below are the three most common and reliable methods for determining the presence and prevalence of varroa mites in your colonies.
Click here for a downloadable PDF of the Standard Operating Procedure (SOP) of an alcohol wash or scroll down for a video and written instructions.
The Alcohol Wash Method
Alcohol Wash:
In this video, we demonstrate how you can monitor your colonies with the Alcohol Wash Method in order to calculate the percentage of varroa mites present and determine if it’s necessary to look into treatments. This method is very similar to the Icing Sugar Shake Method, however, the main difference is that your bees will not survive this method.
What you will need:
• Mite Shaker (for example a double jar mite shaker or an Easy Check). Alternatively, you can use a mason jar with a #8 wire mesh lid.
• A rectangular tub big enough to shake frames into.
• ½ a cup of live bees.
• 70% alcohol or windshield washer fluid with ethanol (enough to cover ½ a cup of bees).
Monitoring:
Add enough 70% alcohol or windshield washer fluid to the mite shaker that it will cover ½ a cup of bees. Shake 1-2 frames of bees into a large rectangular bucket (make sure the queen is not on either frame). Scoop up ½ a cup of bees and pour them into the mite shaker. Close the lid tightly and shake the container for at least 3 minutes. Swirl the mite shaker, leaving the bees on the top and letting the fluid drain into the bottom of the jar. Count the number of mites in the fluid.
Calculating:
There are approximately 300 bees in ½ a cup. Divide the number of mites you counted by 300 and multiply the outcome by 100 to determine what percentage of varroa mites are present in your colonies. If it’s over 1%, it would be to your economic advantage to treat your colonies before their population grows!
The Sugar Shake Method
Icing Sugar Shake:
In this video, we demonstrate how you can monitor your colonies with the Icing Sugar Shake Method in order to calculate the percentage of varroa mites present and determine if it’s necessary to look into treatments. The Icing Sugar Shake Method is widely used because it doesn’t kill your bees, it’s quick, reasonably accurate, and allows for a calculated estimate of mite infestation.
What you will need:
• A mason jar with a #8 wire screen as a lid
• A rectangular tub big enough to shake frames into
• ½ a cup of live bees
• 2-4 teaspoons of icing sugar
• Spray bottle filled with water
• A light-colored tub or platter
Monitoring:
Shake 1-2 frames of bees into a large rectangular tub (make sure the queen is not on either frame). Scoop half a cup of bees and pour them into the mite shaker. Close the lid tightly and add 1-2 teaspoons of icing sugar through the wire mesh lid. Swirl the jar gently to coat the bees with icing sugar and leave the jar under the sun for 3 minutes. Turn the jar upside down and shake the icing sugar (and mites) through the wire mesh onto the light-colored platter. Repeat this process by adding an additional 1-2 teaspoons of icing sugar into the mite shaker. Empty the second batch of sugar onto the platter and use the spray bottle to spray water onto the platter to dissolve the sugar. Take note of how many mites are present. Open the mite shaker and release your sugar-coated bees back into the hive - they’ll get a nice treat while they clean themselves.
Calculating:
There are ~300 bees in half a cup. Divide the number of mites you counted by 300 and multiply the outcome by 100 to determine what percentage of varroa mites are present in your colonies. If it’s greater than or equal to 1%, it would be to your economic advantage to treat your colonies before their population grows!
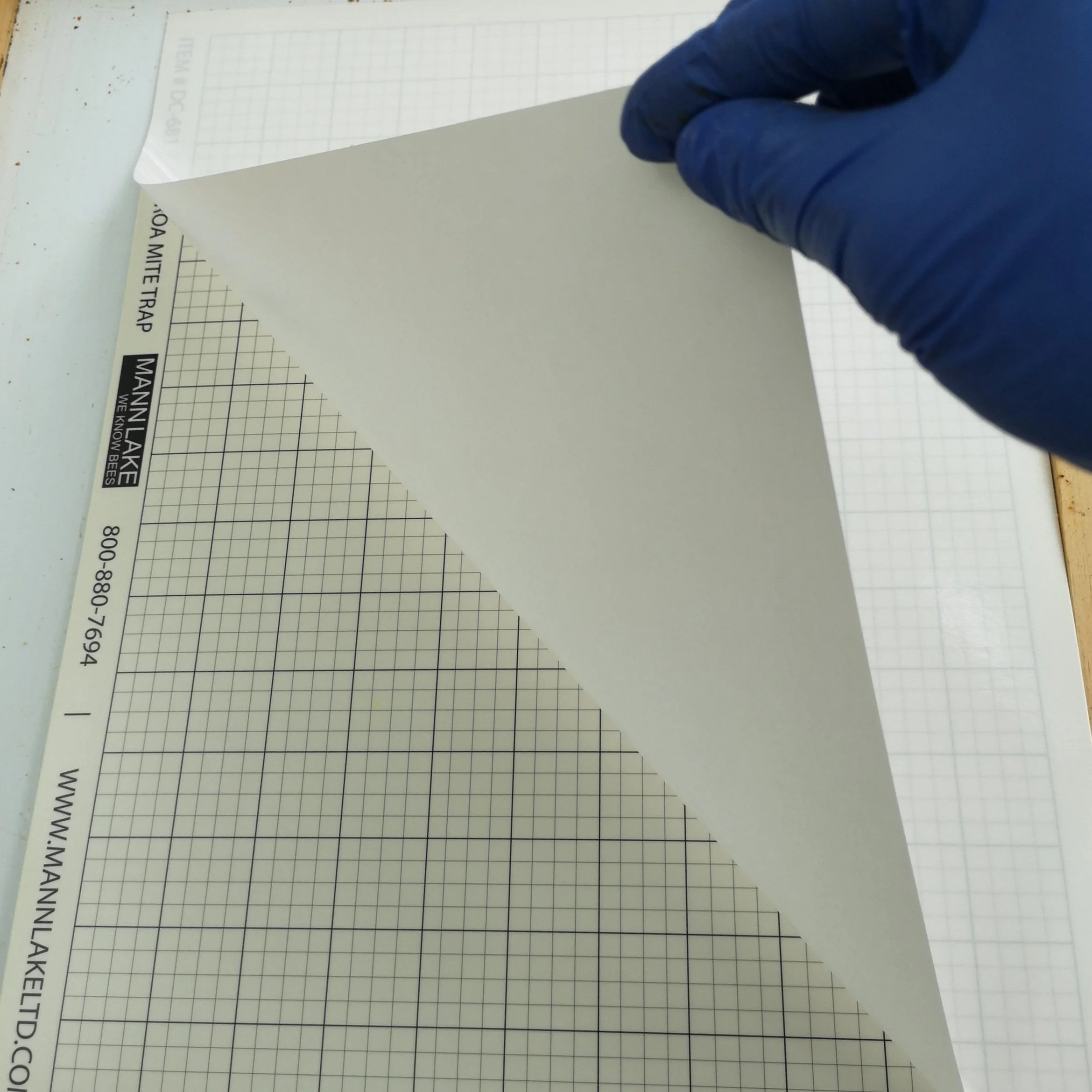
Sticky Board Method
Sticky Board Method
Note: You can purchase commercial sticky boards rather than make your own if preferred.
What you will need:
Heavy paper (e.g., file folder)
Black marker
Sticky material (e.g., vegetable oil or petroleum jelly)
Screened bottom board
Magnifying glass (optional)
Record book and pencil
Monitoring:
Cut the heavy paper to match the size of the screened area of the bottom board (e.g., 15”x18”). Use the black marker to draw a grid on the paper. This will make counting the fallen mites easier (this step is optional). Write the yard, colony number, and date at the edge of the paper. Coat the gridded heavy paper with the adhesive you chose (e.g., vegetable oil or petroleum jelly). Be sure to put enough paste that the paper will remain sticky throughout the duration of the monitoring period. Place the sticky board onto a removable surface (e.g., a tray) under the screened bottom board beneath the brood chamber (most screened bottom boards come with a tray). Recent studies have shown that using screen bottoms boards is not only helpful in counting mites, but that it might be an effective cultural control method to reduce mite levels (Juri et al., 2024). Close the back of the screened bottom board with a wooden insert or tape to prevent insects from getting trapped in the paste (this depends on the design of your screen bottom board). Leave the sticky boards for 3 days (72 hours). After the designated monitoring time, collect the papers and count the varroa mites that are present. Record your results and throw out the used sticky paper.
Calculating:
Divide the number of varroa mites by 3 and this will give you the number of mites that have fallen per 24 hours for that colony (72 hours/24 hours is 3 days). If there are 9-12 mites that have fallen in a 24-hour period, you should treat your colonies for mites (Ontario, 2022).
Calculate varroa growth
Conduct Mite Level Assessment in Spring
Perform a thorough evaluation of mite infestation levels at the beginning of the season (spring).Conduct Mite Level Assessment in Fall
Reassess mite levels approximately 16 weeks after the spring monitoring, during the fall.Determine Mite Population Growth
Calculate the increase in mite levels by subtracting the spring percentage from the fall percentage. This provides an estimate of varroa population growth over the season.
Note: This monitoring approach is particularly recommended for bee breeders aiming to assess varroa resistance or susceptibility in their stock. While treatments may be applied before the spring assessment and after the fall assessment, it is strongly advised not to treat during the 16-week interval. Avoiding mid-season treatments allows for a more accurate evaluation of natural mite population growth and colony resilience.
If you’re interested in learning more, check out the references below.
References:
De la Mora, A., Goodwin, P. H., Morfin, N., Petukhova, T., & Guzman-Novoa, E. (2025). Diversity of potential resistance mechanisms in honey bees (Apis mellifera) selected for low population growth of the parasitic mite, Varroa destructor. Insects, 16(4), 385. Doi: https://doi.org/10.3390/insects16040385
Juri, P., Nogueira, E., Anzola, J., Rodriguez-Batista, V., Branchiccela, B., & Invernizzi, C. (2024). Evaluation of three different bottom boards in honeybee hives for the control of Varroa destructor. Frontiers in Bee Science, 2. Doi: https://doi.org/10.3389/frbee.2024.1384846.
Low Varroa Growth (LVG). (2022). Honey Bee Research Centre. Retrieved from: https://hbrc.ca/low-varroa-growth-lvg/
Ontario. (2022). Varroa mites. Retrieved from the Government of Ontario website: https://www.ontario.ca/page/varroa-mites.
Van Alten, A., Tam, J., & Bryans, R. (2013). Integrated Pest Management for Beekeeping in Ontario. Ontario Beekeepers’ Association Technology Transfer Program.
///
If you have any questions, please send us an email at info@ttp-bchpa.ca or a message through Instagram (@BC_TTP) or Facebook (@bcttp).